The Centers for Medicare and Medicaid Services (“CMS”) made three hospice-focused announcements during July of 2023 indicating the agency is focusing heavily on investigating and preventing hospice fraud and abuse. The government is exercising more oversight over hospice ownership, physician enrollment, and medical directors who certify the need for hospice services.
Hospice patients are a vulnerable population. CMS and the Department of Justice (“DOJ”) place special emphasis on protecting vulnerable patients. A dozen hospice agencies have entered into Corporate Integrity Agreements with the Office of Inspector General since 2015. The DOJ has negotiated 40 hospice-related settlements totaling $266.5 million since 2012. The DOJ has also brought many cases against operators for physician kickbacks, a central feature of hospice fraud investigations.
Merida Group
- 4/21/2021: Operations manager Jose Garza was sentenced to 27 months for a kickback scheme. Witnesses testified that physicians were bribed with illegal payments under the guise of medical directorships to certify unqualified patients. (Source)
- 2/3/2021: Hospice CEO Henry McInnis was sentenced to 15 years for paying kickbacks disguised as directorships to certify ineligible patients for hospice and home health. (Source)
- 12/16/2020: Owner Rodney Mesquias was sentenced to 20 years and ordered to pay $120 million for a similar conspiracy.
(Source)
Allstate Hospice and Verge Home Care
1/19/2021: Founders Onder Ari and Sedat Necipoglu paid $1.8 million to resolve allegations of improper payments to physicians—medical director payments that exceeded fair market value for the actual services provided.
(Sources: Justice; Hospice News)
Compassionate Care Hospice Group
7/6/2017: Compassionate Care Hospice Group, Inc. agreed to pay $2.4 million for allegations involving improper financial relationships with contracted physicians.
Medicare’s 2024 Hospice Proposed Rule
Medicare’s 2024 Proposed Rule includes a new requirement that all physicians certifying hospice services must be enrolled in Medicare or validly opt out, strengthening program integrity. The 2024 Hospice Proposed Rule proposes to require all physicians who order or certify hospice services for Medicare beneficiaries also be enrolled in Medicare or validly opt out. All Medicare-covered hospice services must be ordered and certified by medical physicians. Medicare has limited ability to verify whether unenrolled physicians are qualified or have adverse histories. Medicare believes that closer vetting of physician status, such as licensure, will prevent some fraud and abuse.
Fair Market Value of Hospice Medical Director Compensation
Hospice physician medical directors are the gatekeepers between Medicare hospice reimbursement and bad actors. Kickbacks are a key focus of many criminal cases brought by the DOJ against hospice operators and hospice medical directors.vi A third-party opinion validating the Fair Market Value of hospice medical director compensation is a defense against fraud and abuse allegations and investigations.
Unlike home health agencies, hospices are required to have a medical director under Medicare’s Hospice Conditions of Participation and Medicare’s Hospice Provider Billing Manual. The need for hospice care must be certified by the hospice medical director for every hospice patient, and the medical director may be identified as a hospice patient’s attending physician.
To be covered, hospice services must be reasonable and necessary for the palliation or management of the terminal illness and related conditions. The individual must elect hospice care and a certification that the individual is terminally ill must be completed by the patient’s attending physician (if there is one), and the Medical Director (or the physician member of the Interdisciplinary Group (IDG)).
Payment for physicians’ administrative and general supervisory activities is included in the hospice payment rates. These activities include participating in the establishment, review and updating of plans of care, supervising care and services and establishing governing policies.
Hospice medical directors provide a vital service. Hospice medical director payment arrangements can be validated with market data and defended by adherence to the Stark exceptions and Anti-Kickback safe harbors for professional service agreements.
Market data on hospice medical director compensation illustrates the relationship of physician compensation and overall hospice agency size in terms of operating expenses and revenues.
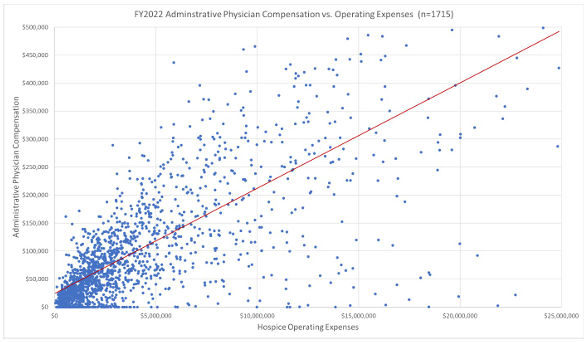
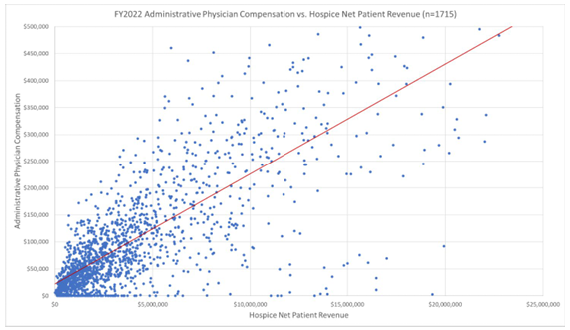
Hourly hospice and palliative care compensation rates are readily available from market surveys and databases. Fair market value analyses should validate both hourly medical director rates for the hospice and palliative care physicians, as well as the overall medical director compensation trends for hospice agencies of similar size. These two factors, fair market value compensation and the commercial reasonableness of the overall compensation arrangement, can be used to fulfill some requirements of the Stark exceptions and Anti-Kickback safe harbors. Hospice agencies should consult their legal counsel to ensure their medical directorships fulfill all requirements of the Stark exceptions and Anti-Kickback Statute safe harbors.
LBMC has helped several hospice agencies develop compliant compensation models for their various medical directors. Fair market value analysis considers agency size and type, the scope of the medical director’s duties and responsibilities, and the specialty and training of the medical director. Hospice agencies can contact LBMC for fair market value and commercial reasonableness opinions to mitigate the risk of being implicated in fraud and abuse investigations.
Nicholas A. Newsad, MHSA works in the Advisory Services Group at LBMC. He can be contacted at nick.newsad@lbmc.com or 615-309-2489.

